As the coffee beans move in the heat of the roaster, hundreds of chemical transformation take place continuously, some of which will decompose, some denatured into other compounds, and countless new compounds formed, all of this plus a series of physical changes will form in the coffee bean unique flavors.
In the language of chemistry, all the reactions involved with coffee in a roaster refer to a process called pyrolysis (endothermically). These are the chemical transformation that occur when organic material reaches the decomposition temperature, which produces volatile compounds and leaves behind a carbon residue (also known as coal). During coffee roasting, we set a limit to avoid charring the beans, but enough for them to undergo chemical changes due to pyrolysis to form flavor compounds…
And here are the main chemical transformation that make up your daily cup of coffee:
1. Maillard’s reaction
Maillard is not just one, but a series of chemical reactions that are crucial to creating the distinctive flavor and brown color of roasted coffee (and many other foods – including chocolate, bread, and coffee). …) The reactions are named after Louis Camille Maillard, the French physician who first described them in 1910. In the roasting oven, the process starts from about 140°C to 160°C, as the temperature rises. Beyond 170°C, the caramelization reactions begin and burn off the remaining sugar.
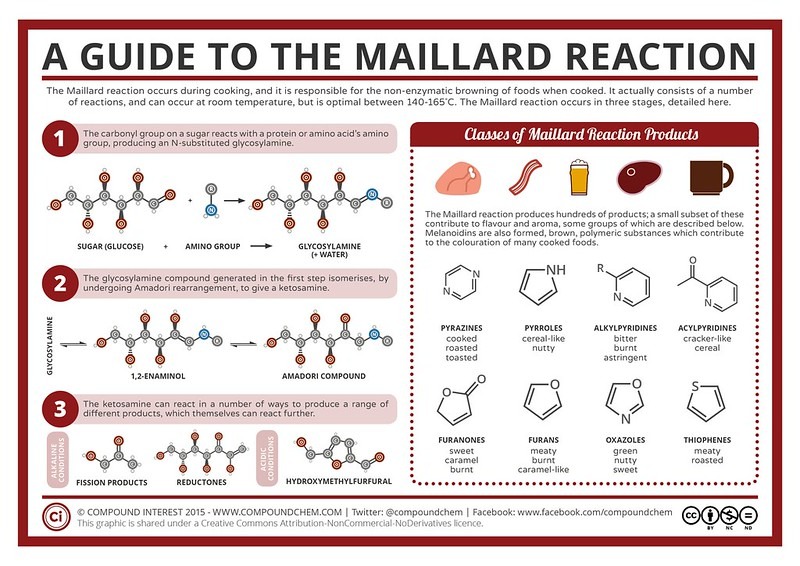
Infographic of Maillard’s reaction
The Maillard reaction occurs between a reducing sugar and an amino acid. A reducing sugar is any sugar with a free aldehyde or ketone group. These groups are attached to the carbon chain via an oxygen atom with a double bond so it can easily react with amino acids and many other compounds.
There are countless ways for the Maillard reaction to occur in coffee beans, with (n) amino acids and (n) sugars giving us n2 different flavor compounds. The most familiar of these products are toasted flavors, toast, etc. What is interesting about the Maillard reaction is that all of its products can react with other free amino acids to form other free amino acids Melanoidin – a dark brown compound, with a roasted, malty, bitter, burnt taste… plays an important role in forming and stabilizing the crema in Espresso, bringing a rich taste to your coffee.
2. Caramelization reaction
Unlike the Maillard reaction, Caramelization is a form of pyrolysis that occurs at about 170°C (338°F). High temperature will cause long, complex carbohydrate chains to break down into hundreds of new, smaller compounds that add a significant amount of bitterness, sourness, aroma, etc. This chemical transformation will take place continuously until the end. roasting process and it also contributes to the sweet aromas in coffee, such as caramel, toast, almonds, etc.

Caramelization plays an important role in the transformation of coffee color and flavor
In addition, it should also be noted that the sucrose content not only gives the sweetness and richness of the coffee after roasting, but also affects the acidity (acidity), because the caramelization of sucrose sugar will produces acetic acid. Therefore, we always encourage coffee growers to harvest the right ripe fruit, with the highest possible sugar content to provide a rich source of raw materials for the Maillard & Caramel reaction team.
A dark roast will break down almost 99% of the grain’s intrinsic sucrose, while a light roast will reduce it by about 87% – Scott Rao
3. Decomposition of acids
Acidity – or the acidic property that gives coffee life, sophistication, complexity and something bright. Although many coffee drinkers claim that acid makes coffee bitter or unpleasant, coffee without acid is actually “empty” and boring. One can experience Cold brew coffee to minimize the acid content in the cup. But that almost negates the roasting process’s incredibly difficult attempt at balancing the acidity.
And when it comes to Acidity, it is impossible not to mention Chlorogenic acid (CGA) by far the most common acid in raw coffee beans, at 6%-8% CGA content in coffee beans is higher than any other type of food any other object. Depending on temperature and time, the roasting process will continuously break down CGA, in light roast 50% of CGA will be lost and only 20% will be left in dark roast. Of course CGAs don’t evaporate, they just break down into quinic and caffeic acids; these two phenolic compounds that contribute to coffee’s bitterness and body (Scott Rao, 2014).
4. Formation of flavor compounds
How many % of solutes are dissolved in a coffee bean?
According to Scott Rao, about a third of the weight of coffee after roasting is water soluble. Meanwhile, the optimal extraction rate is about 19% – 22%; equivalent to about 55% -60% of the total soluble matter in coffee; (because we limit the dissolution of unwanted flavors in cup of coffee); plus a small amount of lipids and broken cellulose fragments (also known as fines). All these solutes, plus the rich aroma, come from one of the following chemical transformation.
4.1. Volatile compounds
A few minutes after the roasting process begins, the new “coffee aroma” is officially formed – A rapid increase in volatile aromatic compounds occurs when the moisture content of the coffee drops below 5%. The Caramelization and Maillard reactions, as well as the degradation and metabolism of amino acids, sugars, phenolic acids and lipids, together contribute to the development of aromatic compounds, including:
– Aldehydes, the scent of green fruits
– Furans, contributing to the caramel flavor
– Pyrazines, with an earthy scent
– Guaiacol, has a smoky, pungent smell
– Sulfur compounds like 2-furfurylthiol give us the characteristic aroma we call “roasted coffee”, but some are not as appealing – for example, methanethiol smells like rotten cabbage.

Coffee will gradually lose its aroma during storage & use
Although CO2 is a volatile compound and does not contribute to the aroma; it plays an important role in the body coffee of coffee in general; and creates the Crema layer in Espresso in particular.
It should note that the aroma content will peak at light-medium roast. Continuing to roast deeper, the heat destroys the aromas created by it, leaving behind a smoky and pungent smell. At the same time, from dark roast onwards, the cellulose structures are weaker and more porous; the ability to retain aroma will be less than the lighter roast.
4.2. Non-volatile compounds
Simply substances that are stable at room temperature. Some of these compounds formed and change during roasting; while others remain stable throughout. Most of the non-volatile compounds contribute to the flavor (flavor) of coffee. For example, caffeine, which is responsible for some of the bitterness, sucrose for sweetness; and lipids for the sensation of body; acids or the melanoidin compounds formed in the Maillard reaction are also non-substances. evaporation…
Speaking of caffeine, despite the common belief, darker roasting only reduces the mass of the beans due to dehydration, not the caffeine content of the beans (since caffeine is more stable at heat than roasting). Therefore, the darker the roast, the more caffeine / weight ratio will increase, so for the same amount of coffee, the darker the roast, the higher the caffeine content.
——————————————————————————————————-
We are here to match your requirement. Do not hesitate to contact us for the best price!
PRINCE COFFEE
ADD: 41/49 Huynh Thuc Khang Street, Dong Da District, HaNoi, VietNam
EMAIL: sales@fineco.com
HOTLINE: +84 966420187 (WHATSAPP/VIBER/ZALO)